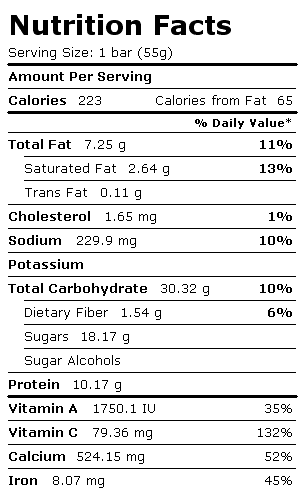
 The two food products that our group has decided on are energy bars and raw bananas.
The two food products that our group has decided on are energy bars and raw bananas.Energy Bar (Multi-Grain)
Raw Banana:
Recommended
Daily Intake (RDI)
The Recommended Daily Intake is the
daily intake of a level of nutrient that is considered enough to meet 97 – 98%
of healthy individuals. In our blog post, we will be focusing on the RDI of
proteins, lipids and carbohydrates. The RDI of the proteins is known to be 50g,
fats or lipids to be 65g, and carbohydrates to be 300g.
As such, in order to meet the RDI using
only energy bars and raw bananas, it would require 9 energy bars and 2 typical
servings of bananas.
Total mass = 9(55) + 2(126) = 747g
Total mass of Carbohydrates = 9(30.32)
+ 2(28.78) = 330.44g
Total mass of Protein = 9(10.17) +
2(1.37) = 94.27g
Total mass of Lipids/Fats = 9(7.25) +2(0.42)
= 66.09g
Proteins
Energy bars contain whey proteins, one
of which being alpha-lactalbumin.
The levels for the structure of the
protein are as followed:
1. Primary structure: the linear sequence
and number of amino acid residues in the polypeptide chain that makes up the
protein. In alpha-lactalbumin, there are 123 amino acid residues.
2. Secondary structure: repetitive folding
or coiling of the polypeptide chain caused by hydrogen bonds formed between the
carbonyl (-C=O) group and imino (-NH) group from the functional groups making
up the peptide bonds.
In
alpha-lactalbumin, the main type of secondary structure involved is the alpha
helix (shown as the thick spirals in the picture above); the alpha helix takes
the form of an extended spiral spring which is stabilised by intramolecular
hydrogen bonds between the O atom of the carbonyl group and the H atom of the imino
group four amino acids away within the polypeptide chain.
3. Tertiary structure: the precise and
compact three-dimensional shape of the protein formed from extensive bending
and folding of the polypeptide chain; this extensive folding and bending is the
result of the 4 different types of interactions between the R-groups of amino
acid residues: ionic bonds, hydrogen bonds, hydrophobic interactions and
disulfide bonds.
In
alpha-lactalbumin, all 4 types of interactions are present.
4. Quaternary structure: the interaction
between two or more polypeptide chains to form an intact and functional
protein.
In
alpha-lactalbumin, there is no quaternary structure.
Enzymes
Raw bananas contain the catabolic
enzyme amylase, which breaks down starch into maltose and glucose in the fruit.
Since enzymes are a type of protein,
their structure follows the levels of protein structure described previously.
Amylase has a primary, secondary (alpha
helices shown as spirals and beta pleated sheets shown as arrows in the picture)
and a tertiary structure.
Beta-pleated sheets is formed when a
single polypeptide chain folds back and forth to form sheets; it is stabilised
by a large number of hydrogen bonds between carbonyl and imino groups of one
part of the backbone and an adjacent part in the parallel regions.
In addition to this, the amino acid
residues that make up the enzyme fall into 4 categories:
1. Catalytic residues which form the basis
of catalytic activity of the enzyme.
2. Contact residues which hold the
substrate (starch in this case) in place.
3. Structural residues that maintain the
correct shape of the active site to ensure the enzyme remains functional.
4. Non-essential residues that do not have
any specific function.
Carbohydrates
In bananas, one type of sugar found is
glucose. Glucose is a simple monosaccharide found in plants, with the formula C6H12O6.
It exists in the open chain form or the cyclic form. The image shown above is
of a cyclic form. In the open chain form, glucose has an unbranched backbone of
6 carbon molecules, with C1 having an aldehyde group. From C2 – C6, they all
contain the hydroxyl groups. The rests of the bonds are with hydrogen atoms.
In both bananas and energy bars, the
sugar found is fructose from the corn syrup. Like glucose, fructose is also a
monosaccharide with the formula C6H12O6. However,
it is bonded differently from glucose, being an isomer of glucose. Fructose is
known to be a ketone, while glucose is an aldehyde. Cyclic fructose consists of
2 CH2OH groups, while glucose only has one CH2OH group.
Lipids
One of the most commonly found lipid in
energy bars is cholesterol. Cholesterol is required for many different
functions in our body, such as maintaining membrane fluidity to being an
important precursor molecule for important hormones in the body. Cholesterol
has a molecular formula of C27H45OH.
In the image above, it shows exactly
what the structure of cholesterol is. The red portion, being the hydroxyl group
(-OH), is the group that determines cholesterol being an alcohol. As the
hydroxyl group is hydrophilic, it makes it soluble in water. The green portion,
being the 4-ring region, is the portion present in all steroid hormones. This
region is made up of hydrocarbons. The blue region is the hydrocarbon tail that
composes of carbon and hydrogen atoms. Both the 4-ring region and hydrocarbon
tail are hydrophobic, thus they will not be soluble in water. As cholesterol
has both hydrophobic and hydrophilic regions, it makes it an amphipathic
molecule.
Thank you for taking the patience to read our blog post! Do feel free to post all your comments :)
Done by:
Sheri Pek
Sheri Pek
Jeanie Quek
Wang Xiao Hui
1SR18
References:
Food Nutrition Labels:
Other Images and Information:










